The Future of Work is in the Cloud. The Future of EDMS is Meridian Cloud for Life Sciences.
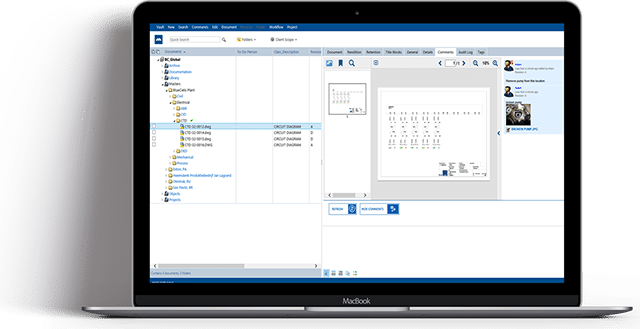
Maximize Security, Simplify Validation & Lower Cost of Ownership with Meridian Cloud for Life Sciences
Meridian’s highly secure, expertly validated SaaS EDMS enables continuous validation
and secure collaboration, helping businesses:

Offload complex validation & compliance processes

Replace outdated, underperforming systems
Maximizing transparency, insights & ROI
Overcome the pain of living in the past with:
Validation as A Service
We take care of system qualification, validations and audits so your team doesn’t have to. That means more resources, fewer security risks, more uptime and fewer interruptions for your organization.
Robust Continuous Compliance Capabilities
Facilitate compliance with Title 21 CFR, cGMP, GMP Annex 11 and other key regulations with MC4LS’ features for electronic signatures, audit logs, controlled document editing and automated watermarks and print stamps.


Next-Level Electronic Document Management
Break down silos, consolidate critical documentation and support collaboration for engineering and manufacturing excellence using online annotations, digital workflow routing, mobile capabilities and more.
Future-Ready Digital Transformation
Top organizations are transitioning to the cloud. Those who get there first will overcome many legacy-related inefficiencies like frequent plant shutdowns, costly validation processes, distribution concerns and more

Contact


